1. Lemp MA, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012 May;31(5):472-478. REF2019OTH4479.
2. Nichols KK, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011 Mar;52(4):1922-1929. RED2022OTH4666.
3. Machalinska A, et al. Comparison of morphological and functional meibomian gland characteristics between daily contact lens wearers and nonwearers. Cornea. 2015 Sep;34(9):1098-1104. REF2022TS4005.
4. Jin X, et al. Hormone replacement therapy benefits meibomian gland dysfunction in perimenopausal women. Medicine (Baltimore). 2016 Aug;95(31):e4268. doi: 10.1097/MD.0000000000004268. PMID: 27495030; PMCID: PMC4979784. REF2022OTH4664.
5. Shamsheer RP, Arunachalam C. A Clinical Study of Meibomian Gland Dysfunction in Patients with Diabetes. Middle East Afr J Ophthalmol. 2015 Oct-Dec;22(4):462-6. doi: 10.4103/0974-9233.167827. PMID: 26692718; PMCID: PMC4660533. REF2022OTH4663.
6. Uzunosmanoglu E, et al. Meibomian gland dysfunction in patients receiving long-term glaucoma medications. Cornea. 2016 Aug;35(8):1112-1116. REF2018TS4020.
7. Cochener B, et al. Prevalence of Meibomian Gland Dysfunction at the time of cataract surgery. J Cataract Refract Surg. 2018 Feb;44(2):144-148. REF2018TS4020.
8. Brooks CC, Gupta PK. Meibomian gland morphology among patients presenting for refractive surgery evaluation. Clin Ophthalmol. 2021;15:315–21. REF2023OTH5017.
9. Duke University website. Immune response likely culprit in eyelid gland condition that causes dry eye. Found at: https://dukeeyecenter.duke.edu/news-events/immune-response-likely-culprit-eyelid-gland-condition-causes-dry-eye, July 2018. Accessed December 10, 2019. REF2020TS4021.
10. American Optometric Association, Paraoptometric Resource Center, CPC Submission, T Petrosyan. Cosmetics and the eye: how your beauty products could be harming your eyes. Found at: https://www.wpa-eyes.org/wp-content/uploads/2018/11/Cosmetics-and-the-Eye.pdf, 2018. Accessed December 10, 2019. REF2020TS4022.
11. Nichols KK, et al. A murine model for characterizing glandular changes in obstructive meibomian gland dysfunction. ARVO. 2014. Abstract #13-A0002. REF2021MLT4010.
12. Schaumberg DA, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011 Mar;52(4):1994-2005. REF2018TS4007.
13. Tomlinson A, et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Diagnosis Subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006-49. REF2019OTH4651.
14. Viso E, et al. Prevalence of asymptomatic and symptomatic Meibomian Gland Dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci. 2012; 53(6): 2601–2606. doi: 10.1167/ iovs.11-9228. 1992;19(12):1950-1954. REF2018OTH4456.
15. DOF2021MLT4009 - TearScienceTM LipiFlowTM (Tahoe Study)I- A Post-Market Evaluation of LipiFlow Treatment in Cataract Surgery Practices CRS. May 18, 2021.
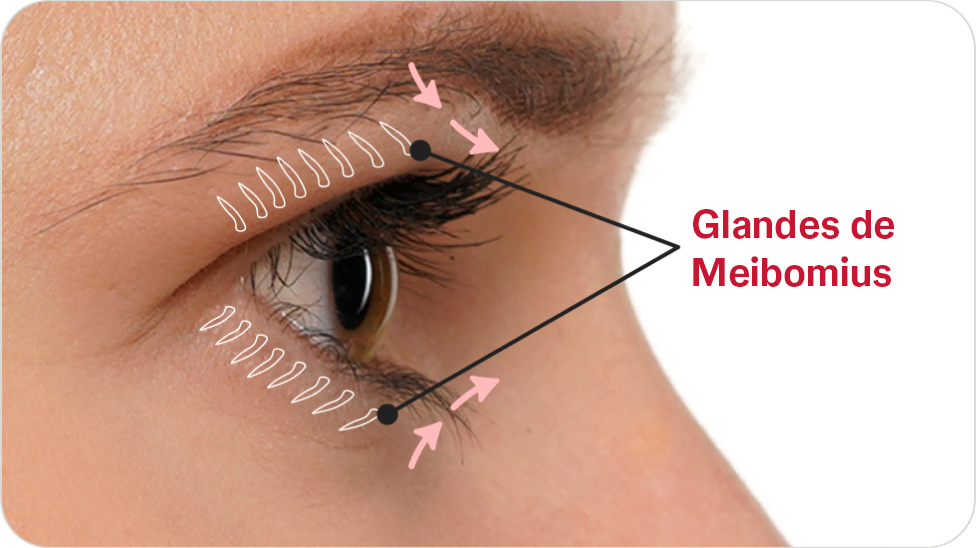
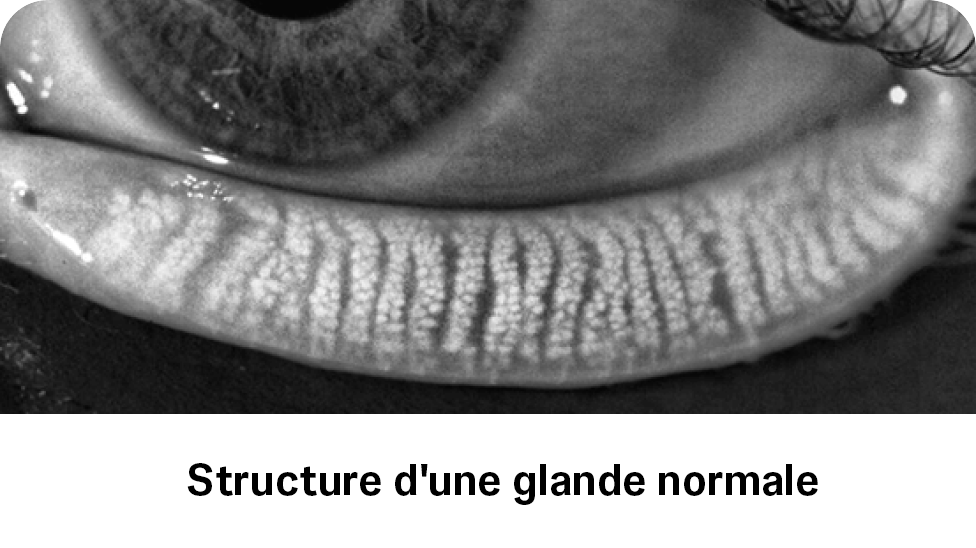
LipiFlowTM est conçu pour l’application d’une thérapie par pression et chaleur localisée chez les patients adultes présentant des conditions de kystes chroniques des paupières, notamment des dysfonctions des glandes tarsiennes (ou glandes de Meibomius - MGD), également désignées sous le terme de sécheresse oculaire par excès d’évaporation ou de syndrome de l’oeil sec par déficience lipidique.
LipiFlowTM est un dispositif médical qui est un produit de santé réglementé et qui porte, au titre de cette réglementation, le marquage CE. Mandataire: Donowa LifeScience Consulting. Ce produit doit être utilisé par des professionnels de la santé uniquement. Pour des informations détaillées sur le produit, y compris les avertissements et les précautions, veuillez consulter le mode d’emploi et consultez votre professionnel de santé.
2024PP11628 Août 2024